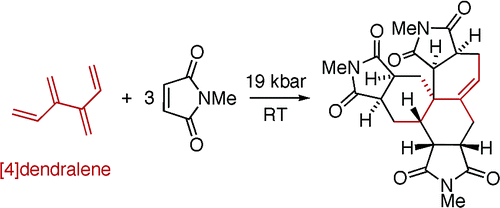
124 Reductive Metallation of Dendralenes and Myrcene using Dimagnesium(I) Compounds: A Facile Route to Unsaturated Organomagnesium Compounds
J. C. Mullins, K. Yuvaraj, M. J. Sowden, M. S. Sherburn, C. Jones
Chem. Eur. J. DOI: coming soon

123 Air Tolerant Cadiot-Chodkiewicz and Sonogashira Cross-Couplings
A. K. K. Fung, M. J. Sowden, M. L. Coote, M. S. Sherburn
Org. Lett. 2023,
DOI: 10.1021/acs.orglett.3c03314

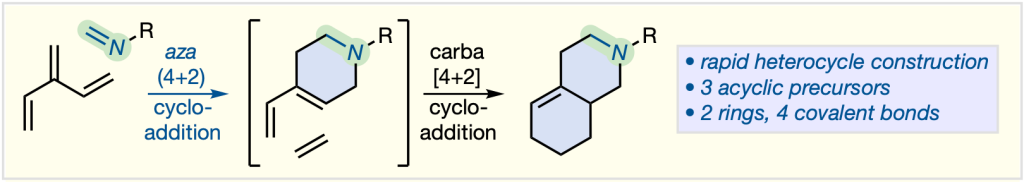
122. A Rapid Aza-Bicycle Synthesis from Dendralenes and Imines
Y.-M. Fan, J. George, J. Y. J. Wang, M. G. Gardiner, M. L. Coote, M. S. Sherburn
Org. Lett. 2023, 25, 7545–7550
DOI: 10.1021/acs.orglett.3c02890
121. Computational and Experimental Confirmation of the Diradical Character of para-Quinonedimethide
Z. Pei, N. L. Magann, M. J. Sowden, R. B. Murphy, M. G. Gardiner, M. S. Sherburn, M. L. Coote
J. Am. Chem. Soc. 2023, 145, 16037–16044
DOI: 10.1021/jacs.3c04363
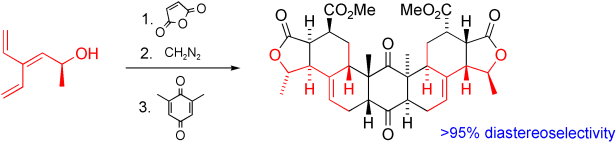
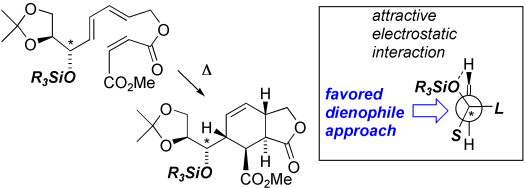
120. Total Synthesis of Matrine Alkaloids
N. L. Magann, E. Westley, M. J. Sowden, M. G. Gardiner, M. S. Sherburn
J. Am. Chem. Soc. 2022, 144, 19695–19699
DOI: 10.1021/jacs.2c09804

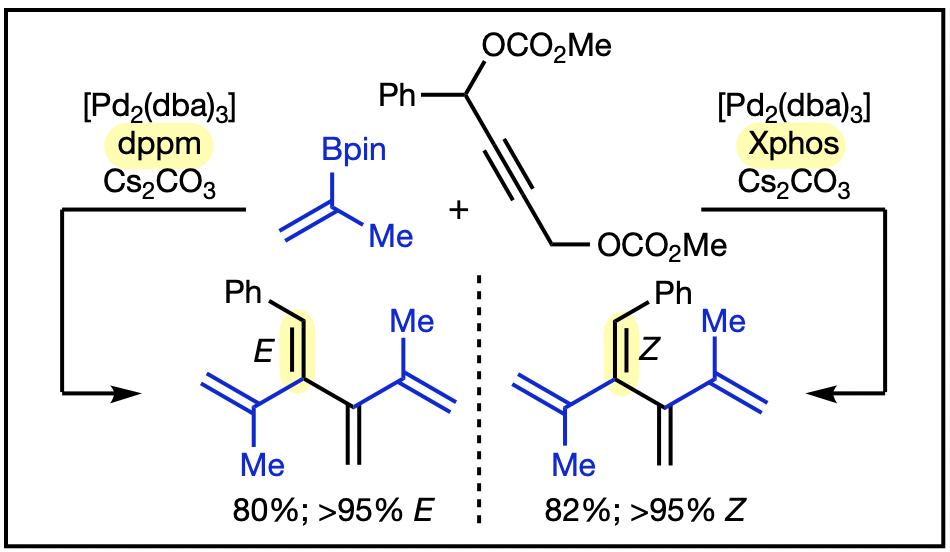
119. A General Stereoselective Synthesis of [4]Dendralenes
Y.-M. Fan, M. J. Sowden, N. L. Magann, E. J. Lindeboom, M. G. Gardiner, M. S. Sherburn
J. Am. Chem. Soc. 2022, 144, 20090–20098
DOI: 10.1021/jacs.2c09360
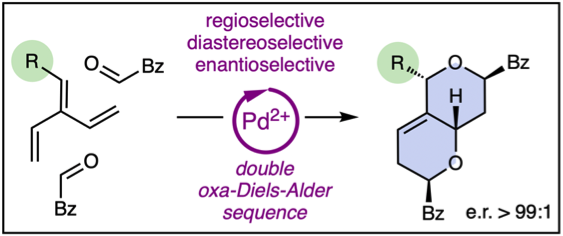
118. Enantioselective oxa-Diels–Alder Sequences of Dendralenes
Y.-M. Fan, L.-J. Yu, M. L. Coote, M. S. Sherburn
Angew. Chem. Int. Ed., 2022, 61, e202204872
DOI: 10.1002/anie.202204872
117 Computational design of next generation atom transfer radical polymerization ligands
M. Stewart, L.-J. Yu, M. S. Sherburn, M. L. Coote
Polym. Chem., 2022, 13, 1067-1074
DOI: 10.1039/D1PY01716K

116 Tuning Photoenolization-Driven Cycloadditions Using Theory and Spectroscopy
J.-Y. Wang, M. Blyth, M. S. Sherburn, M. L. Coote
J. Am. Chem. Soc. 2022, 144, 1023–1033
DOI: 10.1021/jacs.1c12174

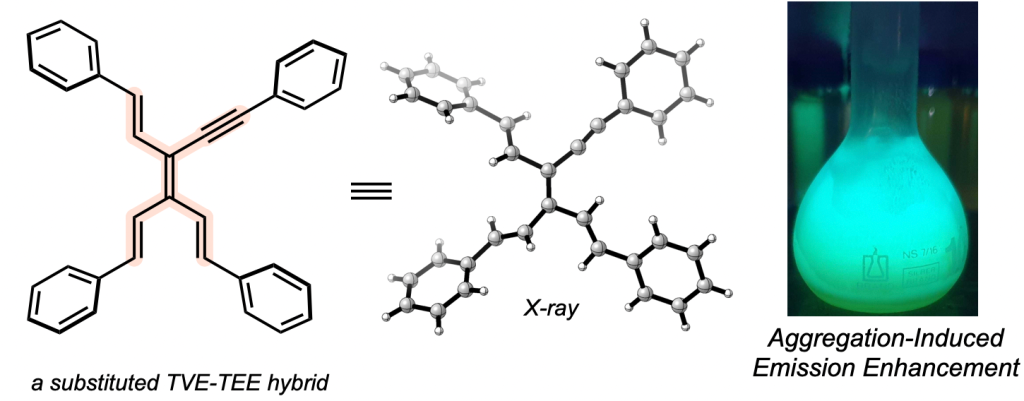
115 Substituted Tetraethynylethylene-Tetravinylethylene Hybrids
E. Westley, M. J. Sowden, N. L. Magann, K. L. Horvath, K. P. E. Connor, M. S. Sherburn
J. Am. Chem. Soc. 2022, 144, 977–98
DOI: 10.1021/jacs.1c11598
114 Allenes in Diels-Alder Cycloadditions
H. Hopf, M. S. Sherburn
Synthesis, 2022, 54, 864-886
DOI: 10.1055/s-0040-1706052

113 Five Step Total Synthesis of Lythranidine
N. L. Magann, M. T. Blyth, M. S. Sherburn
Angew. Chem. Int. Ed., 2021, 60, 18561-1856
DOI: 10.1002/anie.202107524

112 ATRP-Inspired Room Temperature (sp3)C–N Coupling
A. Fung, L.-J. Yu, M. S. Sherburn, M. L. Coote
J. Org. Chem., 2021, 86, 9723–9732
DOI: 10.1021/acs.joc.1c01029

111 Preparation of a Z-Iodoalkene through Stork-Zhao-Wittig Olefination, Stereo-retentive Lithium–iodine Exchange and Z-Boronic acid Pinacol Ester Synthesis
M. A. Morin, S. Rohe, C. Elgindy, M. S. Sherburn
Org. Synth., 2020, 97, 217-223
DOI: 10.15227/orgsyn.097.0217
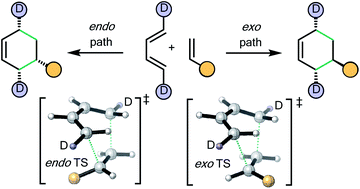
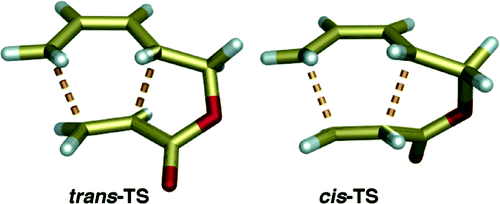
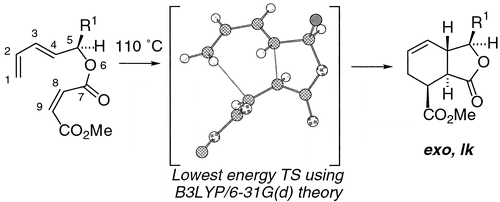
110 The simplest Diels–Alder reactions are not endo-selective
W. J. Lording, T. Fallon, M. N. Paddon-Row, M. S. Sherburn
Chem. Sci., 2020, 11, 11915-11926
DOI: 10.1039/D0SC04553E
109 Synthesis and Properties of 2,3‐Diethynyl‐1,3‐Butadienes
M. S. Sherburn, M. J. Sowden, J. S. Ward
Angew. Chem. Int. Ed., 2020, 59, 4145-4153
DOI: 10.1002/anie.201914807
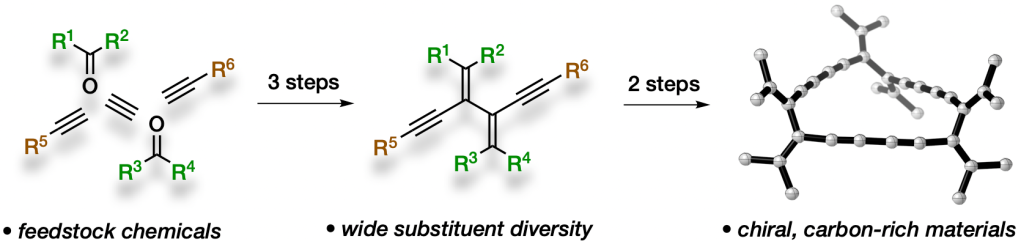
108 Unlocking Acyclic π-Bond Rich Structure Space With Tetraethynylethylene–Tetravinylethylene Hybrids
K. L. Horvath, N. L. Magann, M. J. Sowden, M. G. Gardiner,
M. S. Sherburn
J. Am. Chem. Soc., 2019, 141, 19746-19753
DOI: 10.1021/jacs.9b08885

107 Diene-Transmissive Enantioselective Diels-Alder Reactions and Sequences Involving Substituted Dendralenes
J. George, M. S. Sherburn
J. Org. Chem. 2019, 84, 14712-14723
DOI: 10.1021/acs.joc.9b02296
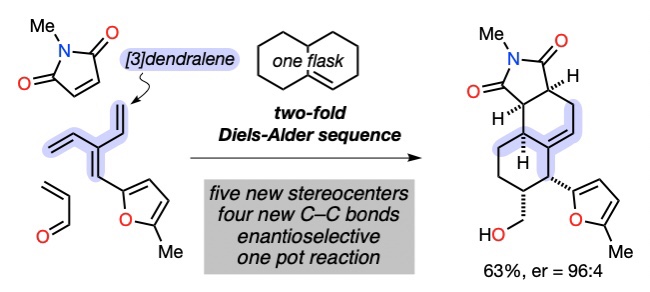
106 Diene-Transmissive Diels–Alder Sequences with Benzynes
J. George, J. S. Ward, M. S. Sherburn
Org. Lett. 2019, 21, 7529-7533
DOI: 10.1021/acs.orglett.9b02807
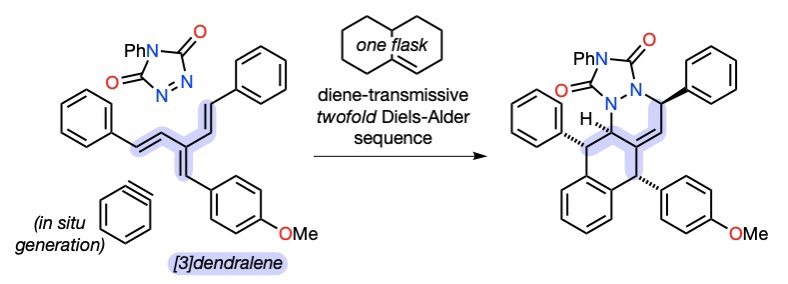
105 A General Synthesis of Dendralenes
J. George, J. S. Ward, M. S. Sherburn
Chem. Sci. 2019, 10, 9969-9973
DOI: 10.1039/c9sc03976g

104 Tetravinylallene
C. Elgindy, J. S. Ward, M. S. Sherburn
Angew. Chem. Int. Ed. 2019, 58, 14573-14577
DOI: 10.1002/anie.201908496
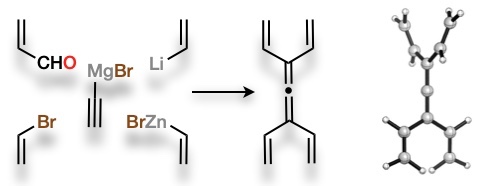
103 A Broad‐Spectrum Synthesis of Tetravinylethylenes
K. L. Horvath, C. G. Newton, K. A. Roper, J. S. Ward, M. S. Sherburn
Chem. Eur. J. 2019, 25, 4072-4076
DOI: 10.1002/chem.201900550

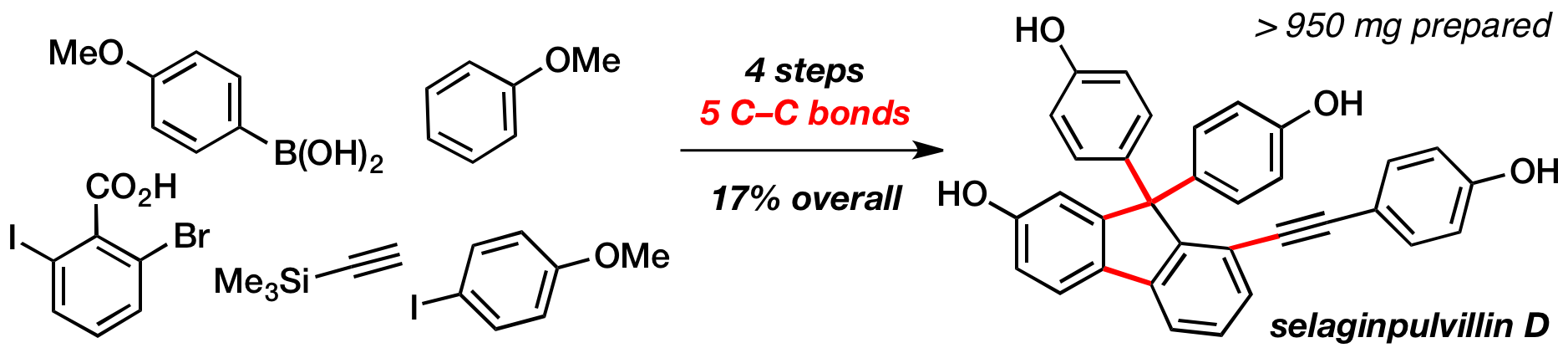
102 Four-Step Total Synthesis of Selaginpulvilin D
M. J. Sowden, M. S. Sherburn
Org. Lett. 2017, 19, 636-63.
DOI: 10.1021/acs.orglett.6b03793
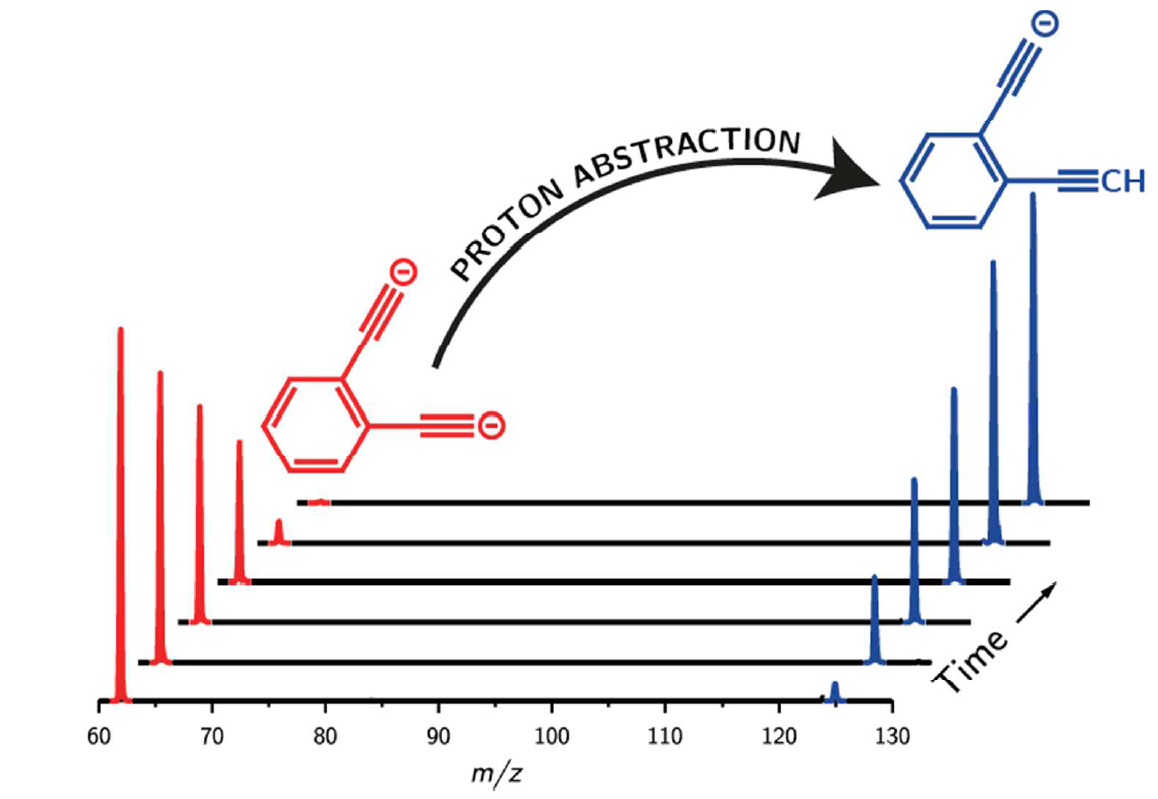
101 Preparation of an Ion with the Highest Calculated Proton Affinity: Ortho-Diethynylbenzene Dianion
B. L. J. Poad, N. D. Reed, C. S. Hansen, A. J. Trevitt, S. J. Blanksby, E. G. Mackay, M. S. Sherburn, B. Chan, L. Radom
Chem. Sci. 2016, 7, 6245-6250.
DOI: 10.1039/C6SC01726F
100 Direct Cross-Couplings of Propargylic Diols
N. J. Green, A. C. Willis, and M. S. Sherburn
Angew. Chem. Int. Ed. 2016, 55,9244 –9248.
DOI: 10.1002/anie.201604527

99 Synthesis and Diels–Alder Reactivity of Substituted [4]Dendralenes
M. F. Saglam, A. R. Alborzi, A. D. Payne, A. C. Willis, M. N. Paddon-Row, and M. S. Sherburn
J. Org. Chem., 2016, 81, 1461-1475.
DOI: 10.1021/acs.joc.5b02583
Selected as the ACS Editor’s Choice open access publication for 29 January 2016
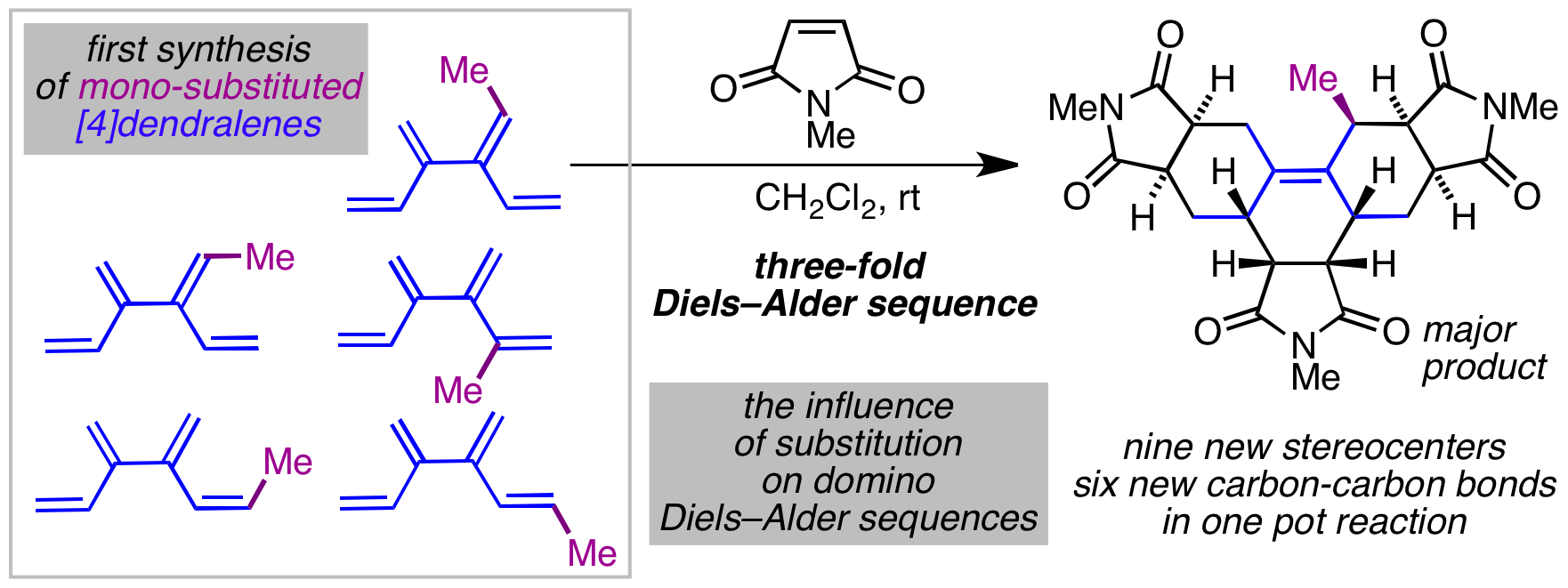
98 Multicomponent diene-transmissive Diels-Alder sequences featuring aminodendralenes
S. M. Tan, A. C. Willis, M. N. Paddon-Row and M. S. Sherburn
Angew. Chem. Int. Ed. 2016, 55, 3081–3085.
DOI: 10.1002/anie.201510925
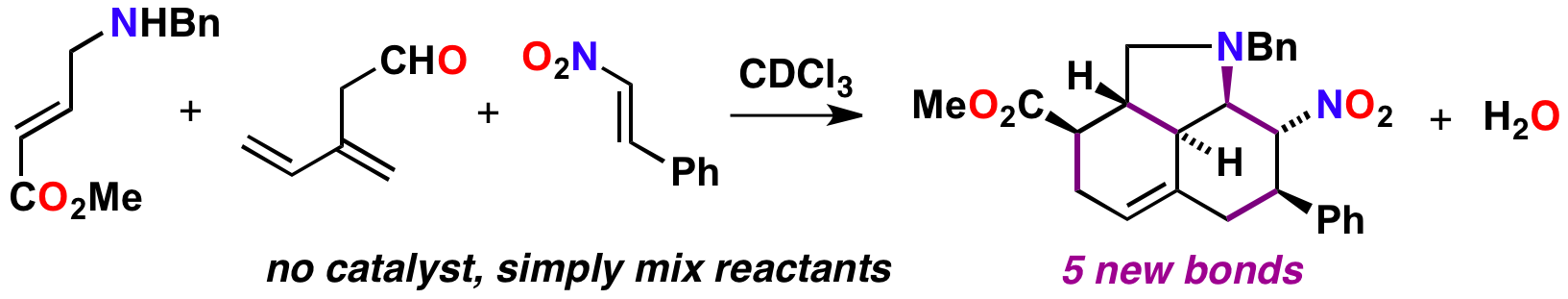
97 Discovery and Computational Rationalization of Diminishing Alternation in [n]Dendralenes
M. F. Saglam, T. Fallon, M. N. Paddon-Row, and M. S. Sherburn
J. Am. Chem. Soc., 2016, 138, 1022-1032.
DOI: 10.1021/jacs.5b11889
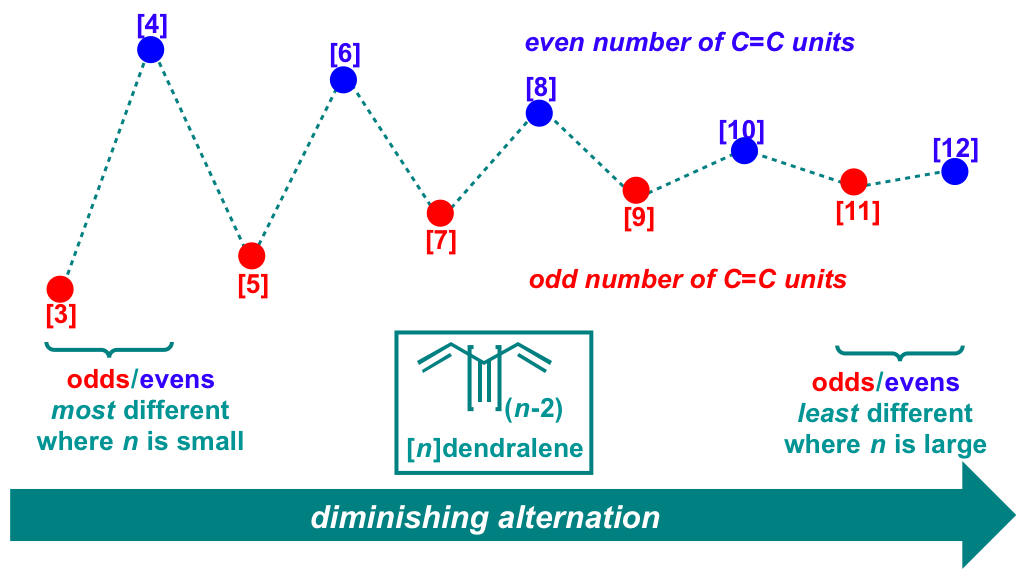
96 A Domino Diels–Alder Approach toward the Tetracyclic Nicandrenone Framework
E. G. Mackay, M. Nörret, L. S.-M. Wong, I. Louis, A. L. Lawrence, A. C. Willis, and M. S. Sherburn
Org. Lett., 2015, 17, 5517–5519.
DOI: 10.1021/acs.orglett.5b02412

95 [5]Radialene
E. G. Mackay, C. G. Newton, H. Toombs-Ruane, E. J. Lindeboom, T. Fallon, A. C. Willis, M. N. Paddon-Row, and M. S. Sherburn
J. Am. Chem. Soc., 2015, 137, 14653–14659.
DOI: 10.1021/jacs.5b07445
Featured on the cover.
![JACS [5]Radialene](https://sherburngroup.files.wordpress.com/2014/05/95.png?w=584)
94 Preparation and Synthetic Value of π-Bond Rich Branched Hydrocarbons
M. S. Sherburn
Acc. Chem. Res., 2015, 48, 1961–1970.
DOI: 10.1021/acs.accounts.5b00242

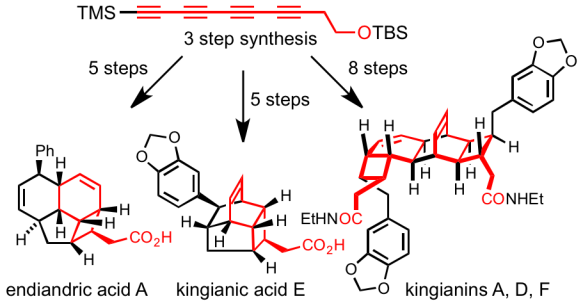
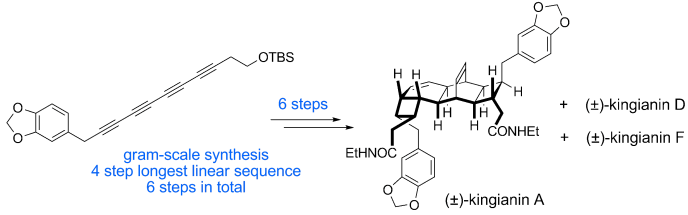
93 Unified Total Synthesis of the Natural Products Endiandric Acid A, Kingianic Acid E, and Kingianins A, D and F
S. L. Drew, A. L. Lawrence and M. S. Sherburn
Chemical Science, 2015, 6, 3886 – 3890.
DOI: 10.1039/C5SC00794A
92 Total synthesis of the pseudopterosin aglycones
C. G. Newton and M. S. Sherburn
Nat. Prod. Rep., 2015, 32, 865-876.
DOI: 10.1039/C5NP00008D

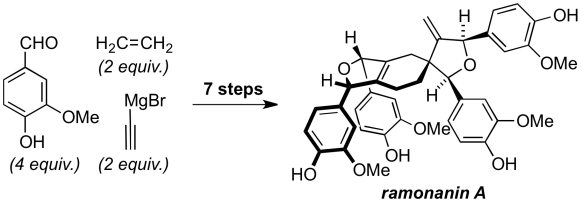
91 Total Synthesis of Ramonanins A–D
R. S. Harvey, E. G. Mackay, L. Roger, M. N. Paddon-Row, M. S. Sherburn, A. L. Lawrence
Angew. Chem. Int. Ed., 2015, 54, 1795–1798.
DOI: 10.1002/anie.201409818
90 A Combined Computational-Experimental Study of the Kinetics of Intramolecular Diels–Alder Reactions in a Series of 1,3,8-Nonatrienes.
W. J. Lording, A. D. Payne, T. N. Cayzer, M. S. Sherburn, M. N. Paddon-Row
Aust. J. Chem. (special issue dedicated to the memory of Dr Des Brown), 2015, 68, 230–240.
DOI: 10.1071/CH14430

89 Pseudopterosin synthesis from a chiral cross-conjugated hydrocarbon through a series of cycloadditions
C. G. Newton, S. L. Drew, A. L. Lawrence, A. C. Willis, M N. Paddon-Row, M. S. Sherburn
Nat. Chem. 2015, 7, 82–86
DOI: 10.1038/nchem.2112

88 The Diels–Alder Reaction in Steroid Synthesis
E. G. Mackay, M. S. Sherburn
Synthesis, 2015, 47, 1–21
DOI: 10.1055/s-0034-1378676

87 Computational and Synthetic Studies with Tetravinylethylenes
E. J. Lindeboom, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
J. Org. Chem., 2014, 79, 11496–11507.
DOI: 10.1021/jo5021294
86 Simple Synthetic Receptors for Aspirin
T. V. Nguyen, M. S. Sherburn
Chem. Eur. J., 2014, 20, 14991–14995
DOI: 10.1002/chem.201304808

85 Tetravinylethylene.
E. J. Lindeboom, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
Angew. Chem. Int. Ed. 2014, 53, 5440-5443
DOI: 10.1002/anie.201402840
84 Furanodendralenes.
T. Fallon, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
J. Org. Chem. 2014, 79, 3185-3193
DOI: 10.1021/jo500458y
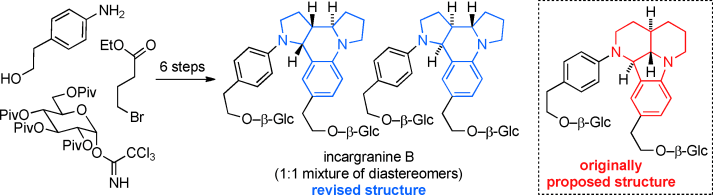
83 Total Synthesis and Structural Revision of the Alkaloid Incargranine B.
P. D. Brown, A. C. Willis, M. S. Sherburn, A. L. Lawrence
Angew. Chem. Int. Ed. 2013, 52, 13273-13275
DOI: 10.1002/anie.201307875
82 Short Synthesis of 3-(Hydroxymethyl)xylitol and Structure Revision of the Anti-diabetic Natural Product from Casearia esculenta.
R. Wang, M. N. Paddon-Row, M. S. Sherburn
Org. Lett. 2013, 15, 5610-5612
DOI: 10.1021/ol402740m

81 Domino Cycloaddition Organocascades.
N. J. Green, A. L. Lawrence, G. Bojase, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
Angew. Chem. Int. Ed. 2013, 52, 8333-8336
DOI: 10.1002/anie.201302185
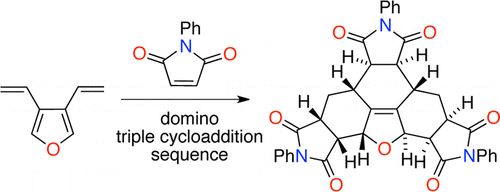
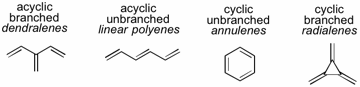
80 Demystifying the Dendralenes. (invited paper)
E. G. Mackay, M. S. Sherburn
Pure Appl. Chem. 2013, 85, 1227-1239
DOI: 10.1351/PAC-CON-13-02-04
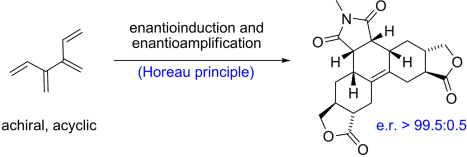
79 Multi-Bond Forming Processes in Efficient Synthesis. Inaugural Beckwith Review (invited review)
N. J. Green, M. S. Sherburn
Aust. J. Chem. 2013, 66, 267-283
DOI: 10.1071/CH13003

78 Total Synthesis of Kingianins A, D, and F.
S. L. Drew, A. L. Lawrence, M. S. Sherburn
Angew. Chem. Int. Ed. 2013, 52, 4221-4224
DOI: 10.1002/anie.201210084
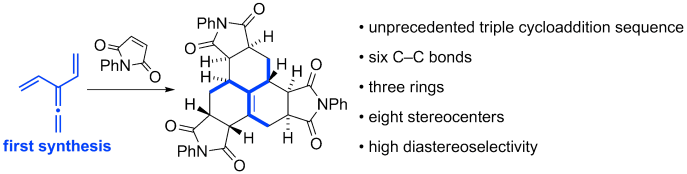
77 Nitroso-dienophile Additions to Dendralenes: A Short Synthesis of Branched Aminosugars.
R. Wang, G. Bojase, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
Org. Lett. 2012, 14, 5652-5655
DOI: 10.1021/ol302619r

76 Total Synthesis of Incarviditone and Incarvilleatone.
P. D. Brown, A. C. Willis, M. S. Sherburn, A. L. Lawrence
Org. Lett. 2012, 14, 4537-4539
DOI: 10.1021/ol302042u

75 On the Diels-Alder Dimerisation of Cross-Conjugated Trienes.
H. Toombs-Ruane , E. L. Pearson , M. N. Paddon-Row, M. S. Sherburn
Chem. Commun. 2012, 48, 6639-6641
DOI: 10.1039/C2CC32520A

74 β-Oligofurans.
T. Fallon, A. C. Willis, A. D. Rae, M. N. Paddon-Row, M. S. Sherburn
Chem. Sci. 2012, 3, 2133-2137
DOI: 10.1039/C2SC20130E

73 On the Origin of the Alternating Diels-Alder Reactivity in [n]Dendralenes.
M. N. Paddon-Row, M. S. Sherburn
Chem. Commun. 2012, 48, 832-834
DOI: 10.1039/C1CC15455A

72 Dendralenes Branch Out: Cross-Conjugated Oligo-Alkenes Allow the Rapid Generation of Molecular Complexity.
H. Hopf, M. S. Sherburn
Angew. Chem. Int. Ed. 2012, 51, 2298–2338
DOI: 10.1002/anie.201102987

71 Basic Concepts on Radical Chain Reactions. Book Chapter
M. S. Sherburn
In Encyclopedia of Radicals in Chemistry, Biology and Materials; C. Chatgilialoglu and A. Studer (Eds.); John Wiley & Sons: Chichester, UK; 2012, pp. 57-80
ISBN: 978-0-470-97125-3

70 1,1-Divinylallene.
K. M. Cergol, C. G. Newton, A. L. Lawrence, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
Angew. Chem. Int. Ed. 2011, 50, 10425–10428
DOI: 10.1002/anie.201105541
69 Synthesis and Applications of Tricarbonyliron Complexes of Dendralenes.
H. Toombs-Ruane, N. Osinski, T. Fallon, C. Wills, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
Chem.—Asian. J. 2011, 6, 3243-3250
doi: 10.1002/asia.201100455

68 Synthesis and Properties of the Ivyanes: The Parent 1,1-Oligocyclopropanes.
G. Bojase, T. V. Nguyen, A. D. Payne, A. C. Willis, M. S. Sherburn
Chem. Sci. 2011, 2, 229-232
doi: 10.1039/c0sc00500b

67 Selective Binding and Release of Aspirin by an Encapsulating Receptor.
T. V. Nguyen, H. Yoshida, M. S. Sherburn
Chem. Commun. 2010, 46, 5921-5923
doi: 10.1039/c0cc00422g

66 Experimental and Computational Studies into an ATPH-Promoted Exo-Selective IMDA Reaction: A Short Total Synthesis of Δ9-THC.
E. L. Pearson, N. Kanizaj, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
Chem.—Eur. J. 2010, 16, 8280-8284
doi: 10.1002/chem.201001176

65 Practical Synthesis and Reactivity of [3]Dendralene.
T. A. Bradford, A.D. Payne, A.C. Willis, M.N. Paddon-Row, M.S. Sherburn
J. Org. Chem. 2010, 75, 491-494
doi: 10.1021/jo9024557

64 Double Dehydro-Diels-Alder Reactions of 1,5-Dien-3-ynes.
T. Fallon, D. E. J. E. Robinson, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
Chem.—Eur. J. 2010, 16, 760-765
doi: 10.1002/chem.200902190

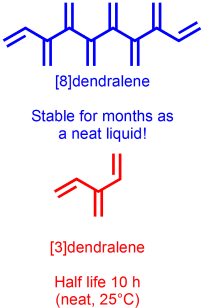
63 Practical Synthesis of the Dendralene Family Reveals Alternation in Behavior. VIP paper
A. D. Payne, G. Bojase, M. N. Paddon-Row, M. S. Sherburn
Angew. Chem. Int. Ed. 2009, 48, 4836-4839
doi: 10.1002/anie.200901733
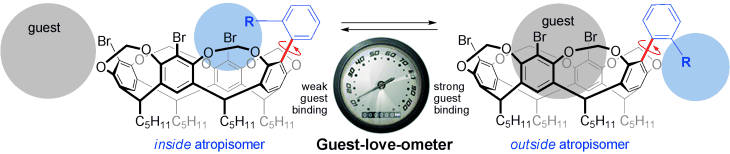
62 Guest Binding Drives Reversible Atropisomerism in Cavitand Hosts.
T. V. Nguyen, D. J. Sinclair, A. C. Willis, M. S. Sherburn
Chem.—Eur. J. 2009, 15, 5892-5895
doi: 10.1002/chem.200900695
61 On the Effect of Tether Composition on cis/trans Selectivity in Intramolecular Diels−Alder Reactions.
M. N. Paddon-Row, A. I. Longshaw, A. C. Willis, M. S. Sherburn
Chem. Asian J. 2009, 4, 126-134
doi: 10.1002/asia.200800352
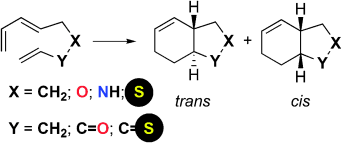
60 Enantioselective Oxazaborolidinium-Catalyzed Diels-Alder Reactions Without CH•••O Hydrogen Bonding.
M. N. Paddon-Row, L. C. H. Kwan, A. C. Willis, M. S. Sherburn
Angew. Chem. Int. Ed. 2008, 47, 7013-7017
doi: 10.1002/anie.200802002
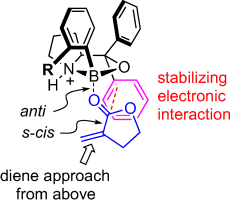
59 Formal Total Synthesis of Triptolide.
N. A. Miller, A. C. Willis, M. S. Sherburn
Chem. Commun. 2008, 1226-1228
doi: 10.1039/b718754h

58 One-Step Synthesis and Exploratory Chemistry of [5]Dendralene.
G. Bojase, A. D. Payne, A. C. Willis, M. S. Sherburn
Angew. Chem. Int. Ed. 2008, 47, 910–912
doi: 10.1002/anie.200704470
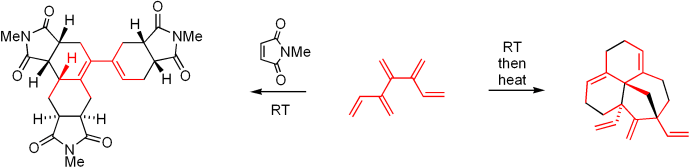
57 Controlling cis/trans-Selectivity in Intramolecular Diels–Alder Reactions of Benzo-Tethered, Ester Linked 1,3,9-Decatrienes.
E. L. Pearson, A. C. Willis, M. S. Sherburn, M. N. Paddon-Row
Org. Biomol. Chem. 2008, 6, 513-522
doi: 10.1039/b716910h
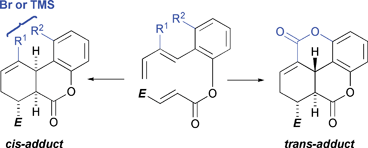
56 Cross-Coupling For Cross-Conjugation: Practical Synthesis and Diels–Alder Reactions of [3]Dendralenes.
T. A. Bradford, A. D. Payne, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
Org. Lett. 2007, 9, 4861–4864
doi: 10.1021/ol7021998

55 Stereocontrol of Intramolecular Diels-Alder Reactions by an Allylic Diphenylcyclopropyl Group.
R. Tripoli, T. N. Cayzer, A. C. Willis, M. S. Sherburn, M. N. Paddon–Row
Org. Biomol. Chem. 2007, 5, 2606-2616
doi: 10.1039/b708324f
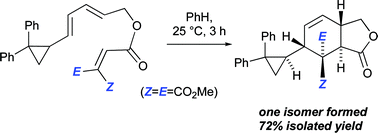
54 Tris(trimethylsilyl)methane is not an Effective Mediator of Radical Reactions.
A. I. Longshaw, M. Carland, E. Krenske, M. L. Coote, M. S. Sherburn
Tetrahedron Lett. 2007, 48, 5585-5588
doi: 10.1016/j.tetlet.2007.06.055

53 Chiral Dendralenes for Rapid Access to Enantiomerically Pure Polycycles.
N. A. Miller, A. C. Willis, M. N. Paddon-Row, M. S. Sherburn
Angew. Chem. Int. Ed. 2007, 46, 937–940
doi: 10.1002/anie.200603335
52 Intramolecular Diels-Alder Reactions of Ester Linked 1,3,9-Decatrienes: cis/trans Selectivity in Thermal and Lewis Acid Promoted Reactions of Ethylene-Tethered and Benzo-Tethered Systems.
E. L. Pearson, L. C. H. Kwan, C. I. Turner, G. A. Jones, A. C. Willis, M. N. Paddon-Row, M S. Sherburn
J. Org. Chem. 2006, 71, 6099–6109
doi: 10.1021/jo0607818

51 Self-Assembly of Supramolecular Platinum Complexes with Bis-4-pyridyl Cavitands.
H. Jude, D. S. Sinclair, N. Das, M. S. Sherburn, P. J. Stang
J. Org. Chem. 2006, 71, 4155–4163
doi: 10.1021/jo060133o

50 Enhanced Stereocontrol in Diels–Alder Reactions of Chiral Dienols.
T. N. Cayzer, N. A. Miller, M. N. Paddon-Row, M. S. Sherburn
Org. Biomol. Chem. 2006, 4, 2019 – 2024
doi: 10.1039/b602618d

49 On the Origin of Cis/Trans Stereoselectivity in Intramolecular Diels-Alder Reactions of Substituted Pentadienyl Acrylates: A Comprehensive Density Functional Study.
M. N. Paddon-Row, D. Moran, G. A. Jones, M. S. Sherburn
J. Org. Chem. 2005, 70, 10841–10853
doi: 10.1021/jo051973q

48 Practical Synthesis and Diels-Alder Chemistry of [4]Dendralene.
A. D. Payne, A. C. Willis, M. S. Sherburn
J. Am. Chem. Soc. 2005, 127, 12188-12189
doi: 10.1021/ja053772+
47 Intramolecular Diels–Alder Reactions of Ester Linked 1,3,8-Nonatrienes.
T. N. Cayzer, M. N. Paddon–Row, D. Moran, A. D. Payne, M. S. Sherburn, P. Turner
J. Org. Chem. 2005, 70, 5561-5570
doi: 10.1021/jo0505829
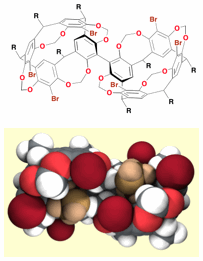
46 Practical Synthesis and Guest-Guest Communication in Multi-hemicarceplexes.
E. S. Barrett, M. S. Sherburn
Chem. Commun. 2005, 3418-3420
doi: 10.1039/b504950d

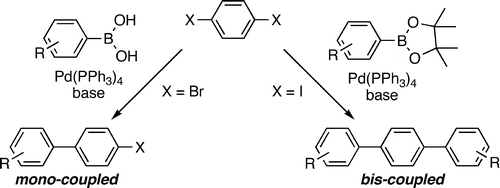
45 Single and Double Suzuki–Miyaura Couplings with Symmetric Dihalobenzenes.
D. J. Sinclair, M. S. Sherburn
J. Org. Chem. 2005, 70, 3730-3733
doi: 10.1021/jo050105q
44 On the Diels–Alder Reactions of Pentadienyl Maleates and Citraconates.
T. N. Cayzer, M. J. Lilly, R. M. Williamson, M. N. Paddon-Row, M. S. Sherburn
Org. Biomol. Chem. 2005, 1302-1307
doi: 10.1039/b501446h

43 Allylic Stereocontrol of the Intramolecular Diels-Alder Reaction.
M. J. Lilly, N. A. Miller, A. J. Edwards, A. C. Willis, P. Turner, M. N. Paddon-Row, M. S. Sherburn
Chem.—Eur. J. 2005, 11, 2525-2536
doi: 10.1002/chem.200401215
42 Double Diels-Alder Reactions of Linear Conjugated Tetraenes.
C. I. Turner, M. N. Paddon–Row, A. C. Willis, M. S. Sherburn
J. Org. Chem. 2005, 70, 1154–1163
doi: 10.1021/jo048108a

41 Superbowl Container Molecules.
E. A. Barrett, J. L. Irwin, A. J. Edwards, M. S. Sherburn
J. Am. Chem. Soc. 2004, 126, 16747-16749
doi: 10.1021/ja044405l

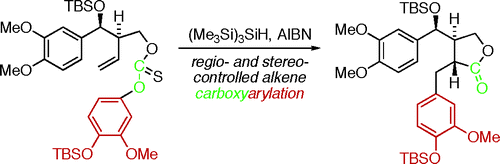
40 Radical Carboxyarylation Approach To Lignans. Total Synthesis of (–)-Arctigenin, (–)-Matairesinol, and Related Natural Products.
J. Fischer, A. J. Reynolds, L. A. Sharp, M. S. Sherburn
Org. Lett. 2004, 6, 1345-1348
doi: 10.1021/ol049878b
39 Chiral Conjoined Cavitands. Invited paper
J. L. Irwin, D. J. Sinclair, A. Edwards, M. S. Sherburn
Aust. J. Chem. 2004, 57, 339-343
doi: 10.1071/CH03299
38 The Intramolecular Carboxyarylation Approach to Podophyllotoxin.
A. J. Reynolds, A. J. Scott, C. I. Turner, M. S. Sherburn
J. Am. Chem. Soc. 2003, 125, 12108-12109
doi: 10.1021/ja0376588

37 IMDA–Radical Cyclisation Approach to Himbacine.
L. S.-M. Wong, M. S. Sherburn
Org. Lett. 2003, 5, 3603-3606
doi: 10.1021/ol0353058

36 Stereocontrol of the Intramolecular Diels–Alder Reaction by Internal Hydrogen Bonding.
T. N. Cayzer, M. N. Paddon–Row, M. S. Sherburn
Eur. J. Org. Chem. 2003, 4059-4068
doi: 10.1002/ejoc.200300414

35 The Domino Intramolecular Diels–Alder Approach to 16-Oxasteroids.
C. I. Turner, R. M. Williamson, P. Turner, M. S. Sherburn
Chem. Commun. 2003, 1610-1611
doi: 10.1039/b303362g
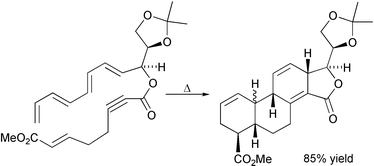
34 On the Endo/Exo Stereoselectivity of Intramolecular Diels-Alder Reactions of Hexadienylacrylates – An Interesting Failure of Density Functional Theory.
G. A. Jones, M. N. Paddon-Row, M. S. Sherburn, C. I. Turner
Org. Lett. 2002, 4, 3789-3792
doi: 10.1021/ol0264713
Erratum: Org. Lett. 2005, 7(20), 4547 10.1021/ol052028r

33 Cavitand Boronic Acids Mediate Highly Selective Fructose Transport.
T. M. Altamore, E. S. Barrett, P. J. Duggan, M. S. Sherburn, M. L. Szydzik
Org. Lett. 2002, 4, 3489-3491
doi: 10.1021/ol0265970

32 The Bromopentadienyl Acrylate Approach to Himbacine.
L. S.-M. Wong, L. A. Sharp, N. M. C. Xavier, P. Turner, M. S. Sherburn
Org. Lett. 2002, 4, 1955-1957
doi: 10.1021/ol0259746

31 Chiral Bis-Cavitand Propellers: Synthesis, Conformations and Multiple Guest Binding.
E. S. Barrett, J. L. Irwin, P. Turner, M. S. Sherburn
Org. Lett. 2002, 4, 1455-1458
doi: 10.1021/ol025704n

30 Partial Etherification Reactions of Cavitand Phenol Bowls.
E. S. Barrett, J. L. Irwin, K. Picker, M. S. Sherburn
Aust. J. Chem. 2002, 55, 319-325
doi: 10.1071/CH02031
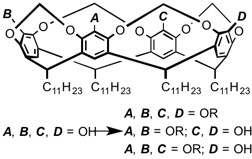
29 Optimising Stereoselectivity in Intramolecular Diels-Alder Reactions of Pentadienyl Acrylates: Synthetic and Computational Investigations into the ‘Steric Directing Group’ Approach.
T. N. Cayzer, L. S.-M. Wong, P. Turner, M. N. Paddon-Row, M. S. Sherburn
Chem.—Eur. J. 2002, 8, 739-750
doi: 10.1002/1521-3765(20020201)8:33.0.CO;2-1

28 Efficient Distal-Difunctionalization of Cavitand Bowls.
E. S. Barrett, J. L. Irwin, P. Turner, M. S. Sherburn
J. Org. Chem. 2001, 66, 8227-8229
doi: 10.1021/10.1021/jo015988+

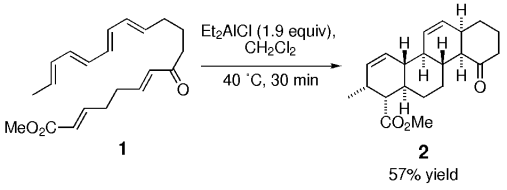
27 The Zipper-Mode Domino IMDA Reaction: A New 0-to-ABCD Strategy for Steroids and Related Compounds.
M. Nörret, M. S. Sherburn
Angew. Chem. Int. Ed. 2001, 40, 4074-4076
doi: 10.1002/1521-3773(20011105)40:213.0.CO;2-J
26 1-(Cholest-4-en-3β-yl)-2,2,2-Trichloroethanimidate tert-Butyl Methyl Ether Hemisolvate.
S. Fielder, D. D. Rowan, M. S. Sherburn, A. K. Burrell
Acta Cryst. 2001, E57, o533-o534
doi: 10.1107/S160053680100825X

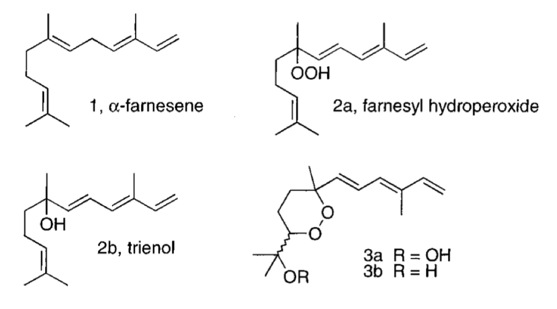
25 Conjugated Triene Oxidation Products of α-Farnesene Induce Symptoms of Superficial Scald on Stored Apples.
D. D. Rowan, M. B. Hunt, S. Fielder, J. Norris, M. S. Sherburn
J. Agric. Food Chem. 2001, 49, 2780-2787
doi: 10.1021/jf0015221
24 Stereocontrol of Intramolecular Diels-Alder Reactions: Synthetic Studies and Transition Structure Modeling with C5-Substituted 1,3,8-Nonatrienes and Nonadienynes.
C. I. Turner, R. M. Williamson, M. N. Paddon-Row, M. S. Sherburn
J. Org. Chem. 2001, 66, 3963-3969
doi: 10.1021/jo015516v
23 Monolithiocavitands: Versatile Intermediates For New Cavitand-Based Hosts.
J. L. Irwin, M. S. Sherburn
Org. Lett., 2001, 3, 225-227
doi: 10.1021/ol006881w

22 The First Synthesis of the [n]Dendralene Family of Fundamental Hydrocarbons.
S. Fielder, D. D. Rowan, M. S. Sherburn
Angew. Chem. Int. Ed., 2000, 39, 4331-4333
doi: 10.1002/1521-3773(20001201)39:233.0.CO;2-3
21 A Density Functional Theory Study of π-Facial Stereoselectivity in Intramolecular Diels-Alder Reactions.
M. N. Paddon-Row, M. S. Sherburn
Chem. Commun. 2000, 2215-2216
doi: 10.1039/B006486F
20 New Insights into the endo-exo Stereoselectivity of the Intramolecular Diels-Alder Reaction of 1,3,8-Nonatrienes.
M. J. Lilly, M. N. Paddon-Row, M. S. Sherburn, C. I. Turner
Chem. Commun. 2000, 2213-2214
doi: 10.1039/B006483L
19 Optimised Synthesis of Cavitand Phenol Bowls.
J. L. Irwin, M. S. Sherburn
J. Org. Chem., 2000, 65, 5846-5848
doi: 10.1021/jo0004456
18 Practical Synthesis of Selectively Functionalised Cavitands.
J. L. Irwin, M. S. Sherburn
J. Org. Chem., 2000, 65, 602-605
doi: 10.1021/jo991185z
17 Synthesis of Sesquiterpene Polyene Hydroperoxides by Regio- and Stereoselective Transposition Reactions.
S. Fielder, D. D. Rowan, M. S. Sherburn
Tetrahedron, 1998, 54, 12907-12922
doi: 10.1016/S0040-4020(98)00782-0
16 The Preparation of α-Farnesene Hydroperoxides for the Study of Superficial Scald.
S. Fielder, M. S. Sherburn, D. D. Rowan
Acta Horticulturae, 1998, 464, 177-181
link: 464_24
15 Stereochemical Control Of The Intramolecular Diels-Alder Reaction By Remote Allylic Substituents On The Diene.
M. J. Lilly, M. S. Sherburn
Chem. Commun., 1997, 967-968
doi: 10.1039/A701125C
14 Synthesis of α-Farnesene Hydroperoxides.
S. Fielder, D. D. Rowan, M. S. Sherburn
Synlett, 1996, 349-350
doi: 10.1055/s-1996-5421
PhD and Postdoctoral Publications
13 Effects Of D-Ring Modified Gibberellins On Flowering And Stem Elongation In Lolium Temulentum.
L. N. Mander, M. S. Sherburn, D. Camp, R. W. King, L. T. Evans, R. P. Pharis
Phytochemistry, 1998, 49, 2195-2206
doi: 10.1016/S0031-9422(98)00310-0
12 The Unusual Structure of a C-Arylated Gibberellin Bis-γ-lactone Formed from a Free Radical-Initiated Cyclisation.
L. N. Mander, M. S. Sherburn, A. C. Willis
Acta Cryst. C, 1997, C53, 223-225
doi: 10.1107/S010827019601147X
11 Unexpected C-Arylation of a Gibberellin: A Cautionary Note on the Radical Deoxygenation of Homoallylic Secondary Alcohols.
L. N. Mander, M. S. Sherburn
Tetrahedron Lett., 1996, 37, 4255-4258
doi: 10.1016/0040-4039(96)00810-6
10 Designer Gibberellins: The Quest for Specific Activity.
L. N. Mander, D. Camp, L. T. Evans, R. W. King, R. P. Pharis, M. S. Sherburn, B. W. Twitchin
Acta Horticulturae, 1995, 394, 45-55
doi: 394_4
9 Stereocontrol in Cyclisation of Dioxolanyl Radicals.
A. Batsanov, M. J. Begley, R. J. Fletcher, J. A. Murphy, M. S. Sherburn
J. Chem. Soc., Perkin Trans. 1, 1995, 1281-1294
doi: 10.1039/P19950001281
8 Stereochemical Control in Reactions of Bicyclic Dioxolanes.
M. J. Begley, R. J. Fletcher, J. A. Murphy, M. S. Sherburn
J. Chem Soc., Chem. Commun., 1993, 1723-1724
doi: 10.1039/C39930001723
7 Intramolecular Free-Radical Substitution of Pyridinium Rings.
J. A. Murphy, M. S. Sherburn
Tetrahedron, 1991, 47, 4077-4088
doi: 10.1016/S0040-4020(01)86445-0
6 A Controlled Balance between Ionic and Radical Pathways in Reactions of Tributyltin Hydride.
J. A. Murphy, M. S. Sherburn, J. M. Dickinson, C. Goodman
J. Chem Soc., Chem. Commun., 1990, 1069-1070
doi: 10.1039/C39900001069
5 Intramolecular Free-Radical Substitution of Pyridinium Rings: Efficient Formation of [5,6] and [6,7]-Fused Ring Systems.
J. A. Murphy, M. S. Sherburn
Tetrahedron Lett., 1990, 31, 3495-3496
doi: 10.1002/chin.199118182
4 Intramolecular Addition of Free Radicals to Quaternised Hereocyclic Rings.
J. A. Murphy, M. S. Sherburn
Tetrahedron Lett., 1990, 31, 1625-1628
doi: 10.1016/0040-4039(90)80034-J
3 A Study of Chemical Reactions Which Report the Presence of Free Radicals.
J. A. Murphy, J. M. Dickinson, M. S. Sherburn, C. W. Patterson, N. F. Wooster
Free Rad. Biol. Med., 1990, 9 (supplement 1), 61
doi: 10.1016/0891-5849(90)90386-W
2 Reaction Mechanisms: Free Radicals.
J. A. Murphy, M. S. Sherburn
Annual Reports B, 1989, 86, chapter 4, part (iii), 73-85
doi: 10.1039/OC9898600073
1 Intramolecular Reactions of Allyloxy Radicals.
A. Johns, J. A. Murphy, M. S. Sherburn
Tetrahedron 1989, 45, 7835-7858
doi: 10.1016/S0040-4020(01)85798-7
